Trypanosomatid parasites of bees
Honeybee health is a major ecological, agricultural and societal concern due to the critical role of these insects for plant reproduction and the massive losses of colonies observed within the last decade. The search for abiotic (e.g. pesticides) and biotic (e.g. pathogens) stressors is essential for understanding bee declines and design protection plans. Eusocial insect colonies, like honeybees, represent ideal “ecological niches” for pathogens, with large numbers of related individuals living in close contact, allowing for rapid disease transmission; this poses challenges to the insects in preventing transmission. Flagellated trypanosomatid parasites including Lotmaria passim and Crithidia mellificae are widely distributed in honeybee colonies and have been associated with colony losses. Our work is focused in understanding how these parasites are distributed in nature as well as the molecular mechanisms use to thrive within the bee host at the molecular level.
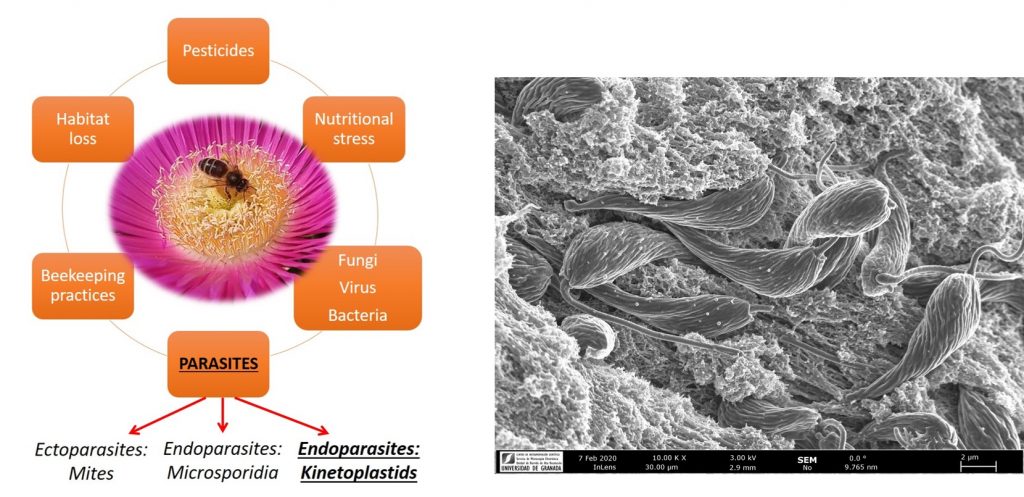
Trypanosomatid Extracellular vesicles
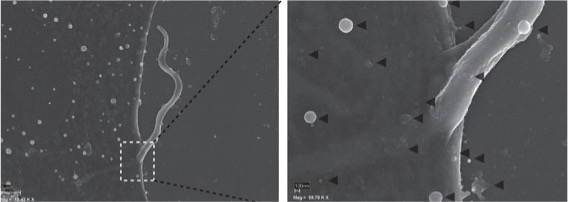
Extracellular vesicles (EVs) are small lipid vesicles released by prokaryotic and eukaryotic cells containing nucleic acids, proteins, and small metabolites essential for cellular communication. Since EVs are virtually released by any cell type, hosts, and pathogens will be constantly shedding and sharing these products to the extra- or intracellular milieu (depending on the niche for proliferation used by the pathogen). In this context, I collaborate with Prof. Antonio Osuna (University of Granada) on the characterisation of EVs from flagellated protozoan parasites belonging to the Order Trypanosomatida. This research involves multidisciplinary approaches from cell biology to proteomics and genomics.
Applications of X-ray microtomography (Micro-CT) in Parasitology Research.
X-ray microtomography or “micro-CT” consists in an x-ray imaging in 3D to resolve structures at small scale (micrometers) with massively increased resolution. Micro-CT involves an x-ray source and detector fixed around a rotating sample generating 2D images from various angles around the object, followed by a digital 3D reconstruction. Our lab is currently working in the development of new protocols for tissue and whole-body 3D reconstruction of samples of parasitic origin (vectors, parasites or hosts), which will be useful to imaging host-parasite interactions at high resolution. This tool that could be exploited by researchers interested in either fundamental or applied biology of parasitic diseases.
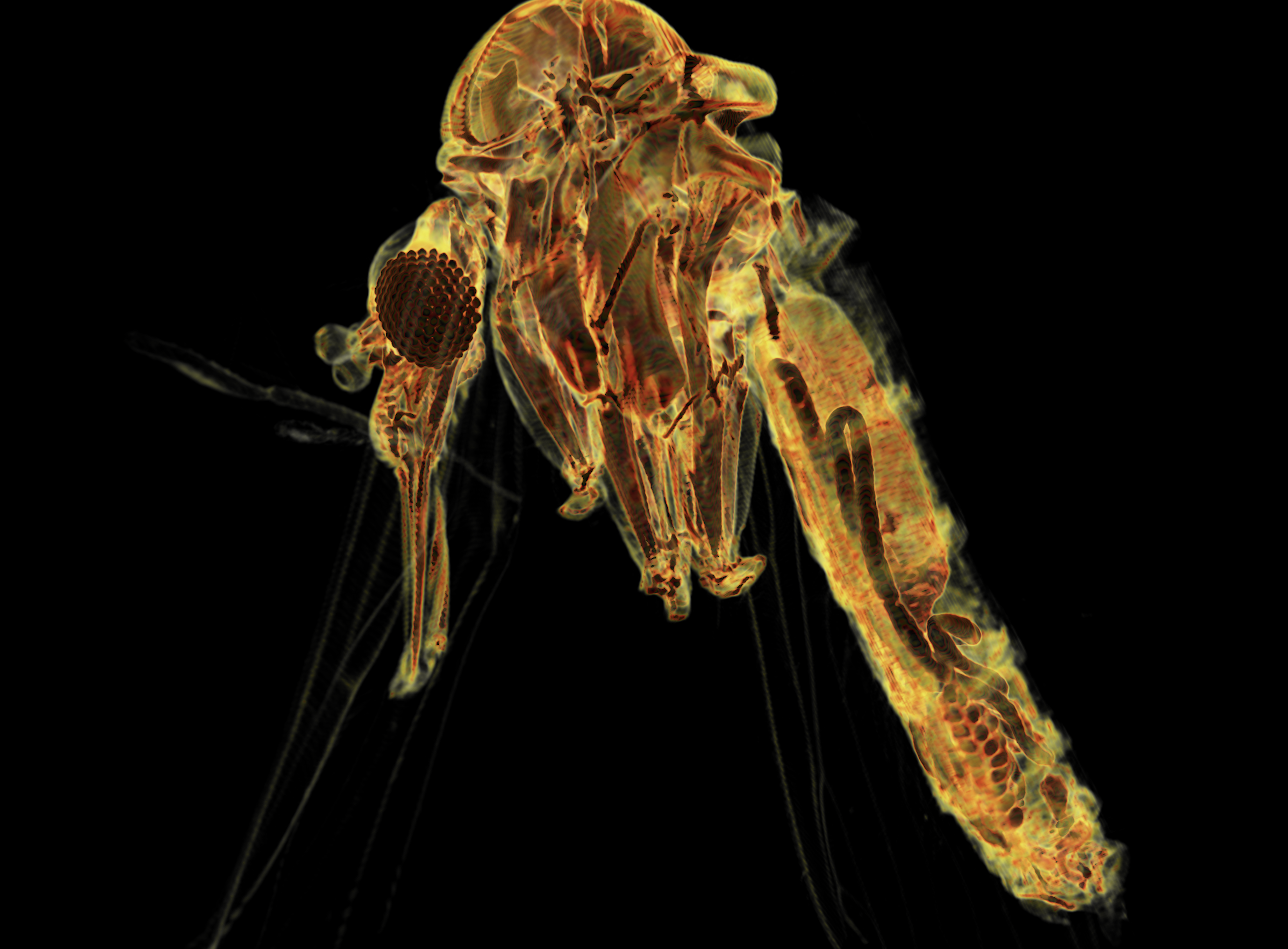
Figure. Full body 3D reconstruction of Phlebotomus ariasi (vector of Leishmania spp.) with highlighted internal anatomy,
My research is supported through several institutions

