- Acknowledgements: The authors gratefully acknowledge the financial support of the Andalusian Government (Junta de Andalucía) for supporting this work (Excellence Project Ref:P12-AGR-1647) and the Spanish Ministry of Economy and Competitiveness (Project ref: AGL2015-70708-R). CTC thanks the predoctoral fellowship associated to the Excellence Project Ref: P12-AGR-1647. MHM wishes to express his appreciation to Fundación Ramón Areces (Spain) for a postdoctoral fellowship.
- Authors: C. Tejada-Casado, M. Hernández-Mesa, F. Monteau, F.J. Lara, M. del Olmo-Iruela, A.M. García-Campaña, B. Le Bizec, G. Dervilly-Pinel
- Reference: Analytica Chimica Acta 1043 (2018) 52-63.
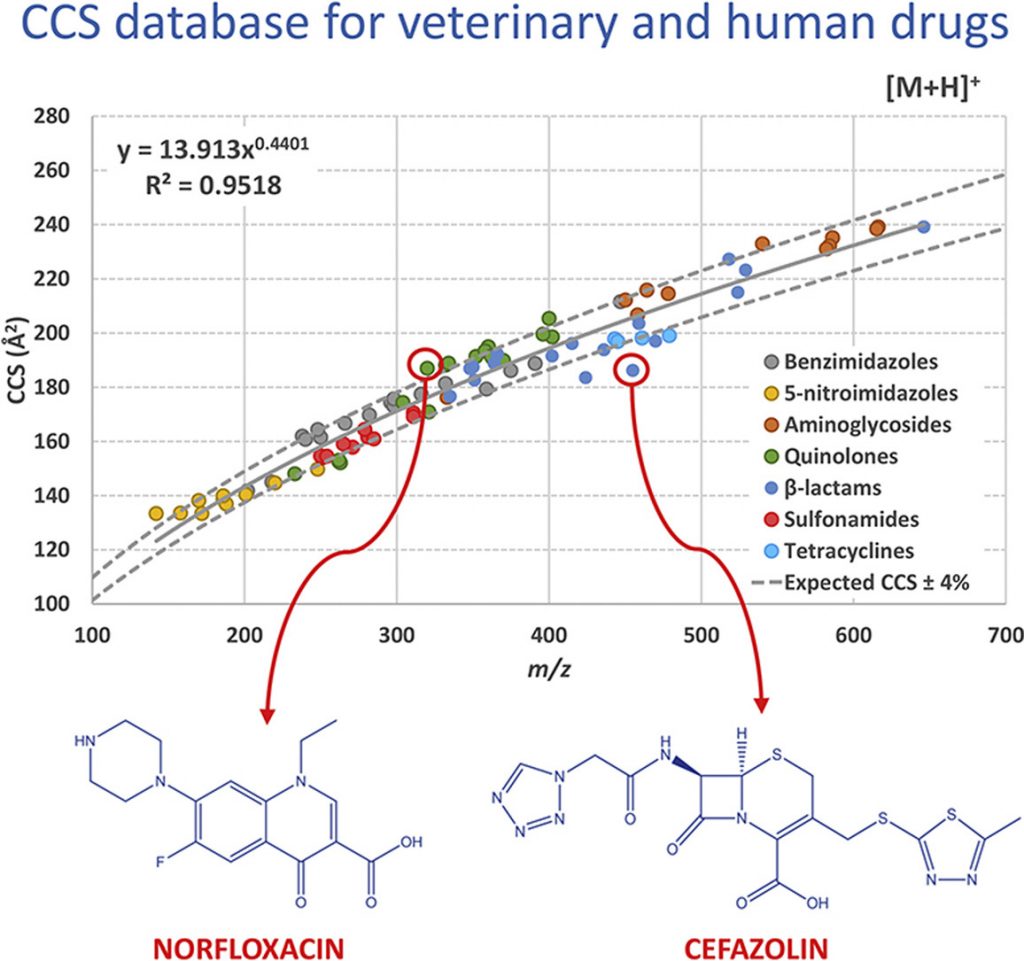
In the context of human and veterinary drugs identification, ion mobility spectrometry (IMS) in combination with mass spectrometry (MS) may provide a relevant complementary piece of information to mass-to-charge ratio (m/z), the so-called collision-cross-section (CCS). Up to now, however, the application of CCS as identification parameter has not been fully investigated due to the reduced number of these drugs that have being characterized in terms of CCS. This work proposes a CCS database for 92 human and veterinary drugs, including eighteen benzimidazoles, eleven 5-nitroimidazoles, eleven aminoglycosides, nineteen quinolones, eighteen β-lactams, ten sulfonamides and five tetracyclines. Among them, 37 drugs have been characterized in terms of CCS for the first time. The CCS values of the other 55 compounds have been compared with those from a recently published database in order to evaluate inter-laboratory reproducibility, which is crucial for the implementation of the CCS as identification parameter. CCS values were measured by traveling wave ion mobility spectrometry (TWIMS) under positive ionization conditions. Nitrogen was used as drift gas in the ion mobility cell. The proposed database covers 173 ions including [M+H]+ and [M+Na]+ species. High correlation between m/z and CCS has been observed for [M+H]+ (R2 = 0.9518, n = 91) and [M+Na]+ (R2 = 0.9135, n = 82) ions. As expected, CCS values for sodium adducts are generally greater than for protonated molecules because they exhibit higher molecular weight. However, sodium adducts of aminoglycosides, β-lactams, and of several quinolones and benzimidazoles, were characterized as more compact ions than their related protonated molecule. In addition, this work describes the fragmentation pattern observed for the studied molecules. For the first time, the main fragment ions for most of the compounds have also been characterized in terms of CCS, involving a total of 238 ions. As proof of concept, for the application of this database to biological matrices, eleven veterinary drugs in bovine urine samples were characterized in terms of CCS, showing that this parameter was not influenced by the matrix.